Office of Human Research Protections Program
Welcome to Wright State University's Institutional Review Board (IRB) website. Here, you will find everything you need to submit a human subjects research study for ethical review.
General Information

Preparing Submissions
![]()
About Us
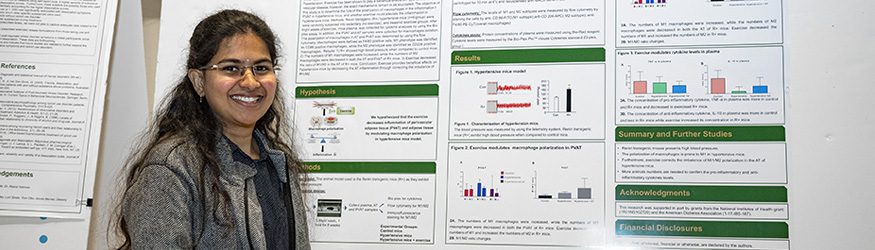
The Human Research Protections Program (HRPP) general information page is a portal to resources, expertise and best practices for researchers, participants, and affiliates.
The HRPP is responsible for managing the Wright State's human research protections program. The HRPP staff provides administrative support to Wright State's Institutional Review Board (IRB). Research involving human subjects must undergo review by the IRB. The IRB is charged with the responsibility of reviewing human subjects research and ensuring compliance with federal regulations, state laws, and Wright State policies. The primary role of the IRB is to protect the safety and welfare of human subjects. The committee is composed of physicians, scientists, non-scientists, and community members with varying backgrounds to promote complete and adequate review of the research activities conducted at Wright State. In addition to working with the IRB, HRPP staff also work directly with investigators and their administrative staff to facilitate submission of the required IRB documentation.
Contact Information
Jennifer Sutton, MPA, CIP
Director, Human Research Protection Program
University Hall 384, 3640 Colonel Glenn Hwy, Dayton, OH 45435-0001
937-775-5171, jennifer.sutton@wright.edu
Chat with me on Microsoft Teams

