Required Reporting During Conduct of Study
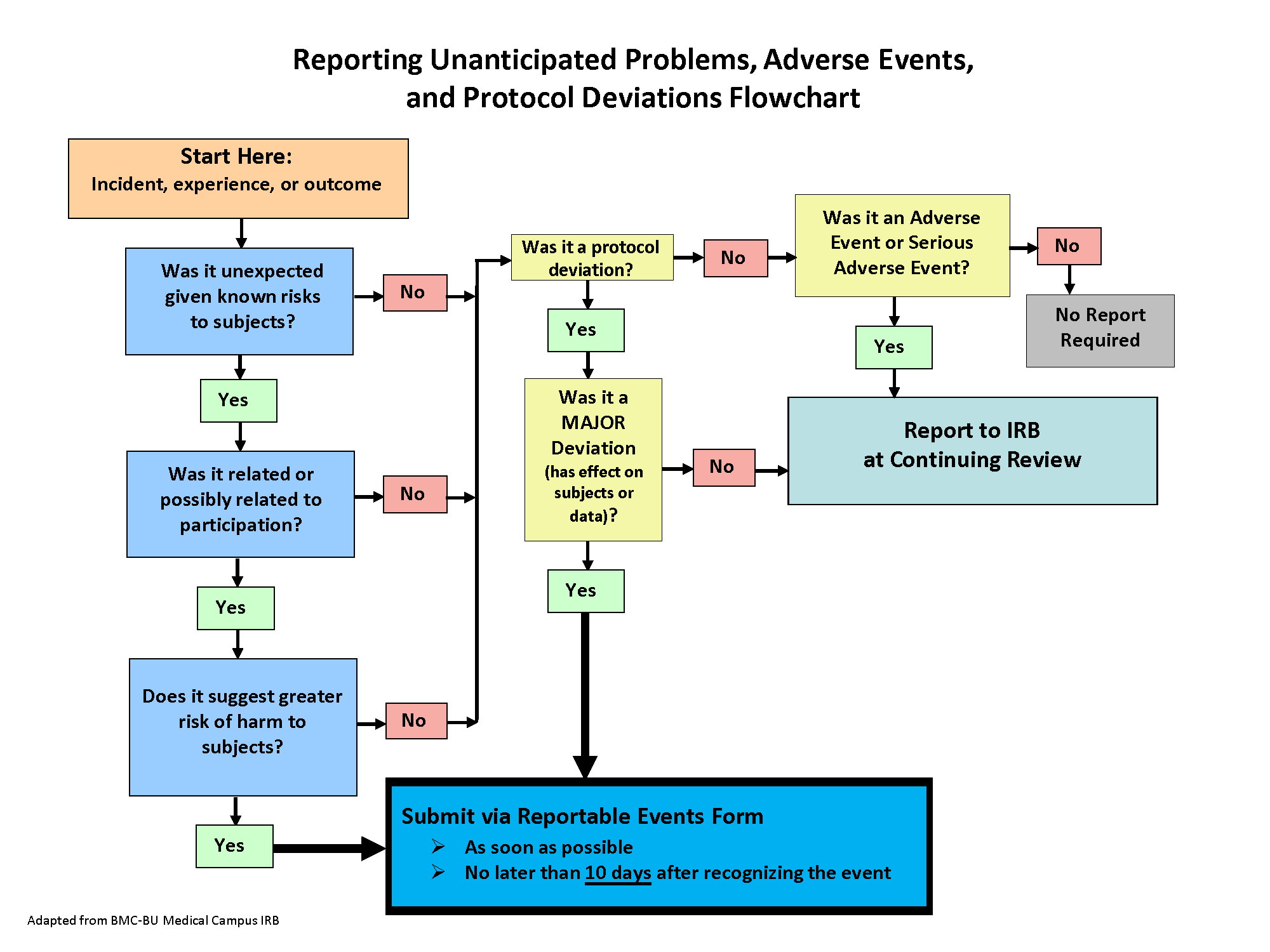
During the conduct of human subject research, investigators sometimes receive information that has a direct impact on the research study, and/or certain types of incidents/events/problems may occur that require prompt review by the IRB. The Incidents, Reportable Problems or Events Policy provides detailed information about the types of incidents/events/problems that must be submitted to the Wright State IRB within 10 business days (see below for examples of events that require prompt reporting). The policy also defines incidents/events/problems that do not require immediate reporting to the Wright State IRB but must be reported at time of renewal/continuing review.
Please consult the policy to determine whether an incident/event that occurred during your study must be reported to the IRB and if needed, add, complete, and submit a form in Cayuse Human Ethics. If the study also requires changes based on the event reported, an modification can be submitted concurrently in Cayuse.
Types of Reportable Incidents:
- Unanticipated Problem or Adverse Event
- Internal or External
- Internal Subject Death even if anticipated if occurs within 30 days of study procedures
- Adverse Device Effects
- Protocol deviation/violation
- Alteration to approved study procedures
- Change in research to eliminate an immediate hazard to a subject
- Report(s) to or from oversight entity
- Report of study lapse
- Accident/incident
- Data Breach
- Self Report of Noncompliance
- Subject Complaint
- Subject Incarceration
- Subject Withdrawal
- Pertinent publication/public announcement
- Notification of audit/inspection/inquiry
Examples of incidents, problems or events that must be promptly reported to IRB:
- A subject event during the research that is serious (death or hospitalization), unexpected (not listed as risk in consent) and related or possibly related to the study
- Subject death in a “greater than minimal risk” study even if expected if it occurs within 30 days of study-related research procedure
- Major deviation from the approved IRB protocol, such as:
- Failure to obtain written consent prior to a subject’s study participation
- Recruiting subjects under the age of 18 when not previously approved by IRB
- Subject received a dose of a study drug that is not consistent with the protocol
- Breach of confidentiality for research involving protected health information (PHI), such as loss of a laptop containing identifiable study data
- Incarceration of an active study subject for a study that is not approved to involve prisoners
- Subject complaint that cannot be fully resolved by the study team
- FDA or sponsor audit (does not include regular sponsor monitoring)
How do I submit an Incident or Reportable Event?
For a step-by-step guide for creating and submitting a reportable event submission in Cayuse, refer to the ![]() Cayuse Investigators Guide. The Information regarding Incident submissions start on slide 65.
Cayuse Investigators Guide. The Information regarding Incident submissions start on slide 65.